Abstract
Background
Peripheral T-cell lymphoma (PTCL) comprises a heterogeneous group of T-cell non-Hodgkin's lymphomas with poor prognosis that are often refractory to treatment. Spleen tyrosine kinase (SYK) and Janus kinase (JAK) signaling pathways are important tumor survival mechanisms in PTCL. Cerdulatinib (ALXN2075) is an orally active, small molecule, reversible ATP-competitive dual inhibitor of SYK/JAK family members. The efficacy and safety of cerdulatinib monotherapy were investigated in a multicenter, single-arm Phase 2a dose-expansion study (NCT01994382) of patients with T- or B-cell malignancies; we report the final results from the PTCL cohort.
Methods
Eligible patients in the PTCL cohort were aged ≥18 years and had histologically confirmed PTCL with relapsed/refractory disease after ≥1 prior systemic therapy (prior brentuximab was required for CD30+ patients) and an Eastern Cooperative Oncology Group performance status ≤1. Patients received oral cerdulatinib 30 mg twice daily in 28-day cycles until disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed overall response rate (ORR) based on Lugano criteria. Secondary endpoints, including time to response (TTR), duration of response (DoR), and progression-free survival (PFS), were estimated. Efficacy was evaluated in patients who had ≥1 post-baseline scan. Safety was evaluated in all patients who received ≥1 dose.
Results
Overall, 220 patients (with various subtypes of B- and T-cell malignancies) were enrolled across 6 study cohorts. A total of 65 patients in the PTCL cohort received ≥1 dose of cerdulatinib and were included in this analysis: 63.1% male; median (range) age 65 (21-85) years; median (range) prior regimens 2 (1-10); 49.2% refractory to last treatment; 18 patients (27.7%) had prior stem cell transplant. Histologic types were angioimmunoblastic T-cell lymphoma/T follicular helper (AITL/TFH [N=29; 44.6%]); PTCL not otherwise specified (NOS [N=11; 16.9%]); and other rare T-cell leukemias and lymphomas (N=25; 38.5%; Table 1).
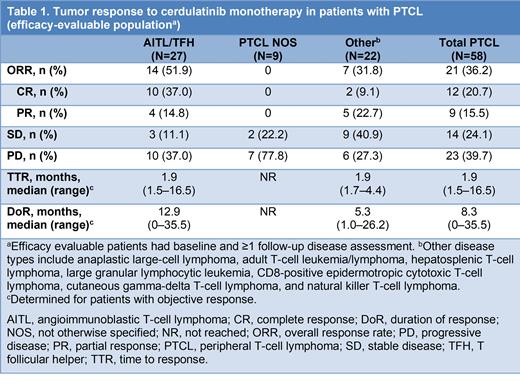
Of 65 patients with PTCL, 58 were evaluable for efficacy (Table 1). ORR was 36.2% (95% CI 24.0-49.9%) for the overall PTCL cohort (12 complete responses [CRs]; 9 partial responses [PRs]; 14 stable disease [SD]). For the AITL/TFH subgroup (N=27), ORR was 51.9% (95% CI 31.9-71.3%) (10 CR; 4 PR; 3 SD). ORR (95% CI) in the safety population was 32.3% (21.2-45.1%) for PTCL overall (N=65) and 48.3% (29.4-67.5%) for AITL/TFH (N=29). Median (range) TTR for AITL/TFH was 1.9 (1.5-16.5) months (first scan at 2 months). Median (range) DoR for AITL/TFH was 12.9 (0.0-35.5) months with median (range) follow-up of 16.4 (0-35.5) months. PFS for the AITL/TFH subgroup was estimated to be median (range) 4.6 (0.6-37.4) months with median (range) follow-up of 22.4 (0.6-37.4) months. Further assessment is warranted.
Median (range) time on treatment for all patients was 2.9 (0.2-37.9) months for PTCL overall and 3.3 (0.5-37.9) months for AITL/TFH, which appears comparable to time patients spent on their last prior therapy (median 2.2 and 3.0 months, respectively). The most common (≥5%) grade ≥3 treatment-emergent adverse events (TEAEs), excluding disease progression, were increased amylase (23.1%), anemia (20.0%), increased lipase (18.5%), neutropenia (12.3%), sepsis (9.2%), and diarrhea (7.7%). Increases in amylase and lipase were transient, reversible, and not associated with clinical pancreatitis. Adverse events were primary reasons for treatment discontinuation in 6 patients (9.2%) in the PTCL population (2 EBV reactivation, 1 West Nile viral infection, 1 sepsis, 1 infection, and 1 colitis).
Conclusions
Cerdulatinib demonstrated acceptable tolerability and clinical activity in PTCL. Complete and durable responses were observed in patients with the AITL/TFH subtype, including patients who had repeatedly relapsed and/or were refractory to their last treatment. These data provide proof of concept and a promising efficacy/safety profile for a first-in-class, dual SYK/JAK inhibitor in relapsed/refractory PTCL. Overall the benefit/risk profile of cerdulatinib treatment appears favorable in this population, with the exception of the PTCL NOS subgroup, in whom no responses were seen. This subtype-specific activity raises the potential for biomarker identification to optimize patient selection.
Horwitz: ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Affimed: Research Funding; Aileron: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; Celgene: Research Funding; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding. Feldman: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Study investigator. Ye: Alexion, AstraZeneca Rare Disease: Other: Study investigator. Khodadoust: Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Other: Study investigator; CRISPR Therapeutics, Nutcracker Therapeutics: Research Funding. Munoz: Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, and Millennium: Research Funding; Alexion, AstraZeneca Rare Disease: Other: Study investigator; Pharmacyclics/Abbvie, Bayer, Kite, a Gilead Company, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa Kirin, Alexion, Fosun Kite, Innovent, Seagen, BeiGene, Debiopharm, Epizyme, Karyopharm, ADC Therapeutics, Servier, and Genmab: Consultancy, Other: advisory role; Targeted Oncology, OncView, Kyowa Kirin, Physicians' Education Resource, and Seagen: Honoraria; Kite, a Gilead Company, Kyowa, Bayer, Pharmacyclics/Janssen, Seagen, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche.: Speakers Bureau. Hamlin: Incyte, Janssen, Molecular Templates: Research Funding; Alexion, AstraZeneca Rare Disease (formerly Portola Pharmaceuticals): Other: Study investigator, Research Funding; Kite, Karyopharm, Celgene: Membership on an entity's Board of Directors or advisory committees. Kim: Portola: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galderma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Elorac: Research Funding; Soligenix: Research Funding; Eisai: Research Funding; Innate: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Corvus: Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; Mundipharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CRISPR: Research Funding; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Secura Bio: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Other: Study investigator; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Horizon: Research Funding. Wilcox: Alexion, AstraZeneca Rare Disease: Other: Study investigator. Patel: ModernaTX: Research Funding; EMD Serono: Membership on an entity's Board of Directors or advisory committees, Research Funding; Evelo Biosciences: Research Funding; GlaxoSmithKline: Research Funding; Bicycle Therapeutics: Research Funding; Mirati Therapeutics: Research Funding; Vigeo: Research Funding; Incyte: Research Funding; Daiichi Sankyo: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Checkpoint Therapeutics: Research Funding; Effector Therapeutics: Research Funding; Forma Therapeutics: Research Funding; H3 Biomedicine: Research Funding; Boehringer Ingelheim: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agenus: Research Funding; Ignyta: Research Funding; Jacobio: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jounce Therapeutics: Research Funding; Klus Pharma: Research Funding; Kymab: Research Funding; Loxo Oncology: Research Funding; LSK Biopartners: Research Funding; Lycera: Research Funding; Placon Therapeutics: Research Funding; Portola Pharmaceuticals: Research Funding; Prelude Therapeutics: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding; Revolution Medicines: Research Funding; Ribon Therapeutics: Research Funding; Seven and Eight Biopharmaceuticals: Research Funding; Syndax: Research Funding; Synthorx: Research Funding; Stemline Therapeutics: Research Funding; Taiho: Research Funding; Takeda: Research Funding; Tesaro: Research Funding; Xencor: Research Funding; Alexion, AstraZeneca Rare Disease: Other: Study investigator; Bayer: Membership on an entity's Board of Directors or advisory committees; ORIC Pharmaceuticals: Research Funding; Eli Lilly: Research Funding; Hengrui: Research Funding; Hutchinson MediPharma: Research Funding; Cyteir Therapeutics: Research Funding; Clovis: Research Funding; Calithera: Research Funding; AstraZeneca: Research Funding; Aileron Therapeutics: Research Funding; Artios Pharma: Research Funding; Gilead: Research Funding; Genentech/Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Exelixis: Membership on an entity's Board of Directors or advisory committees; Vedanta: Research Funding; Curis: Research Funding; Ciclomed: Research Funding; TopAlliance: Research Funding; Macrogenics: Research Funding; Verastem: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; BioNTech: Research Funding; Millennium Pharmaceuticals: Research Funding; Merck: Research Funding; Mabspace: Research Funding; ADC Therapeutics: Research Funding; Acerta Pharma: Research Funding; Florida Cancer Specialists: Research Funding; Phoenix Molecular Designs: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees. Coffey: Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in publicly-traded company. Osman: Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in publicly-traded company. Holland: Alexion, AstraZeneca Rare Disease: Consultancy. Guzman: Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in publicly-traded company. Smith: Alexion, AstraZeneca Rare Disease: Other: Study investigator; Celgene, Genetech, AbbVie: Consultancy.
Final safety and efficacy results from the PTCL cohort treated with cerdulatinib (ALXN2075); an orally active, small molecule, reversible ATP-competitive dual inhibitor of SYK/JAK family members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal